| Structural formula | 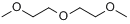 |
| Uses |
Aprotic polar solvent, can be used in a polar organic reactions, anionic polymerization, ion coordination polymerization solvent ;can be used for reduction, alkylation and condensation reaction as solvent, also as Grignard and similar synthetic media, also as a non-polluting cleaning agent, extraction agent, diluent, medical aid and resin solvent. |
| Molecular formula |
C6H14O3 |
| Molecular weight | 134.18(134.17) |
| CAS | 111-96-6 |
| EINECS RN | 203-924-4 |
| InChI | 1S/C6H14O3/c1-7-3-5-9-6-4-8-2/h3-6H2,1-2H3 |
| Appearance | colorless transparent liquid with an ether smell |
| Boiling point(101.3kPa) | 159.76℃(162s℃) |
| Freezing point | —64.04 |
| Specific gravity(25℃/4℃) | 0.9467 |
| Refractive index(25℃) | 1.4097(1.407-1.409) |
| Flash point | 60℃(close). 63℃(open) (57℃) |
| Melting point | -64~-68℃(-64℃) |
| Density | 0.9440 g/mL(0.937) |
| Surface tension(mN/M)20℃ | 31.1 |
| Solubility | Can be miscible with water, alcohol, ether, hydrocarbons |
| Viscosity(20℃) | 0.981mPa.s(25)、1.06(20) |
| Chemical properties |
colorless liquid having an ether smell; stable property, not easy to react; , decomposition occurs in the presence of an acidic catalyst at high temperature; with oxygen to form Peroxide. |
| Purity(GC)% | ≥99.5 |
| Moisture% | ≤0.1 |
| Acidity(as HAC)% | ≤0.020(0.015) |
| Peroxide(as H2O2)% | ≤0.005 |
| Packing | 190kg |






